
(Image credit: Photo12/Universal Images Group via Getty Images) (opens in new tab) Mendeleev's first Periodic Table of Elements is shown here.
"The problem from the historian's perspective is that while Mendeleev kept almost every document and draft that crossed his hands after he believed he would become famous, he did not do so before his formulation of the periodic law."
Periodic table of elements full#
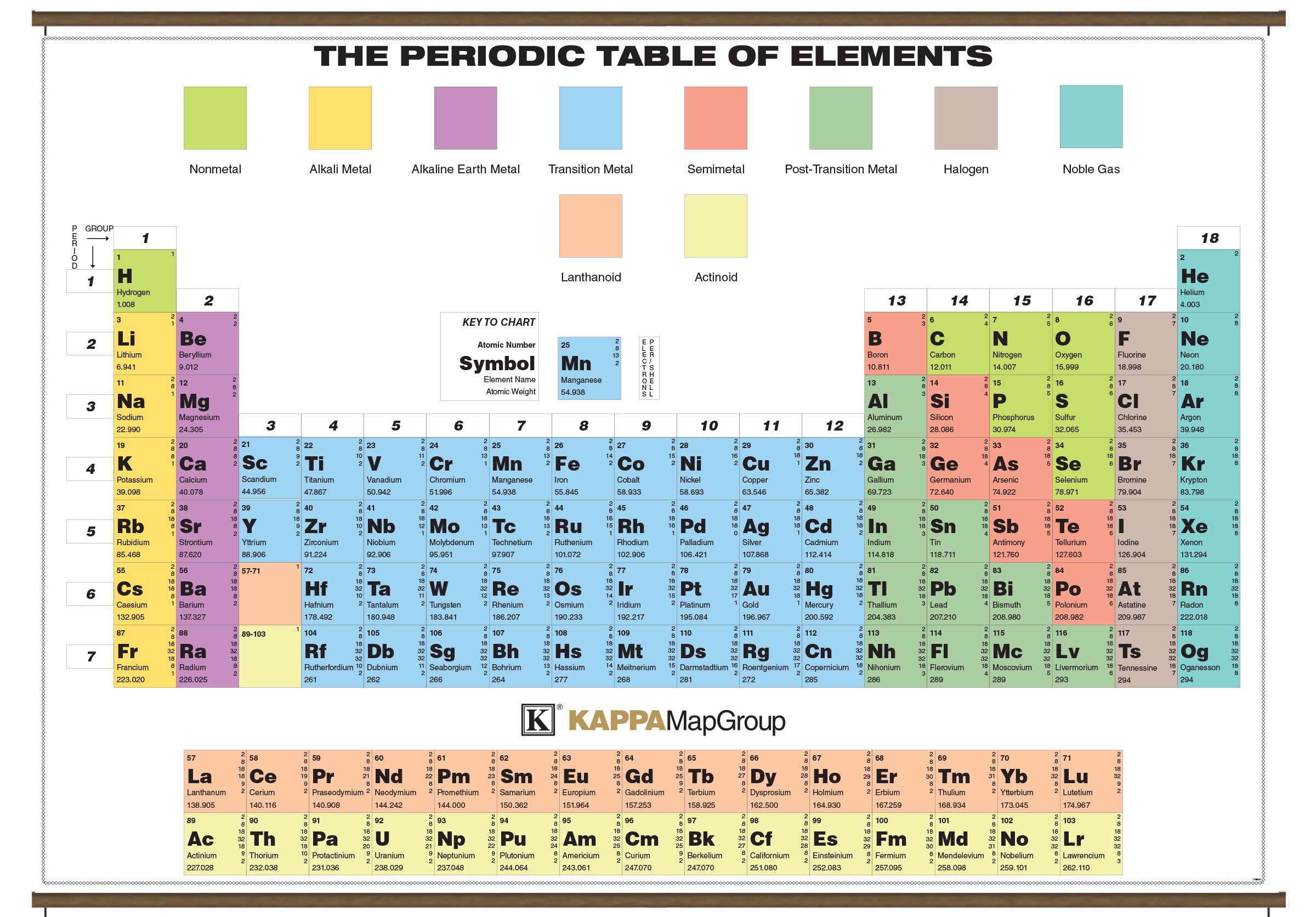
"It is extremely difficult to reconstruct the process by which Mendeleev came to his periodic organization of elements in terms of their atomic weights," Gordin wrote of the full periodic table. This is when he noticed certain types of elements regularly appearing and noticed a correlation between atomic weight and chemical properties.īut the exact Eureka! moment that led Mendeleev to the sorting strategy that produced his complete periodic table is shrouded in mystery. So according to the Royal Society of Chemistry, Mendeleev wrote the properties of each element on cards, and then he started ordering them by increasing atomic weight. But they weren't enough to usefully sort the 55 additional chemical elements known at the time. Gordin in his book "A Well-Ordered Thing: Dmitrii Mendeleev and the Shadow of the Periodic Table" (Princeton University Press, Revised Edition 2018). The first section of Mendeleev's book dealt with just eight of the known elements - carbon, hydrogen, oxygen, nitrogen, chlorine, fluorine, bromine and iodine - and those two strategies worked for those particular elements, according to Michael D. Just two strategies existed at the time to categorize these elements: separating them into metals and nonmetals or grouping them by an element's number of valence electrons (or those electrons in the outermost shell). At the time, there were 63 known chemical elements, each with an atomic weight calculated using Avogadro's hypothesis, which states that equal volumes of gases, when kept at the same temperature and pressure, hold the same number of molecules. Putting the elements in any kind of order would prove quite difficult. (Image credit: Oxford Science Archive/Print Collector/Getty Images) For instance, all the group 18 elements are inert gases, meaning they don't react with any other elements. Elements that occupy the same column on the periodic table (called a "group") have identical valence electron configurations and consequently behave in a similar fashion chemically. As an example, elements in Group 8A (or VIIIA) all have a full set of eight electrons in the highest-energy orbital, according to chemist William Reusch, on his webpage at Michigan State University (opens in new tab). The columns, or groups, on the periodic table represent the atomic elements that have the same number of valence electrons, or those electrons in the outermost orbital shell. (Atoms have protons and neutrons in their nucleus, and surrounding that, they have their electrons arranged in orbitals, where an atomic orbital is a math term that describes the location of an electron as well as its wave-like behavior.)įor instance, period 1 includes elements that have one atomic orbital where electrons spin period 2 has two atomic orbitals, period 3 has three and so on up to period 7. The horizontal rows on the periodic table are called periods, where each period number indicates the number of orbitals for the elements in that row, according to Los Alamos National Laboratory (opens in new tab).


 0 kommentar(er)
0 kommentar(er)
